 GlaucoNova Labs
GlaucoNova LabsGlaucoN va Labs
va Labs
Ultrasound-Integrated Smart Glasses Revolutionizing
Eyecare at Low-Costs

























The Problem
Problem Framing:
Glaucoma is the leading cause of irreversible blindness, affecting 80 million individuals worldwide, and it's estimated that one in every 200 remains undiagnosed. It has a global economic burden of $411 billion. The foremost biomarker is elevated intraocular pressure (IOP), the buildup of aqueous humor damaging optic nerves. Causes underlying this phenomenon are unknown, there exists no cure, and it typically lacks warning symptoms, leading to gradual and "silent" vision loss.
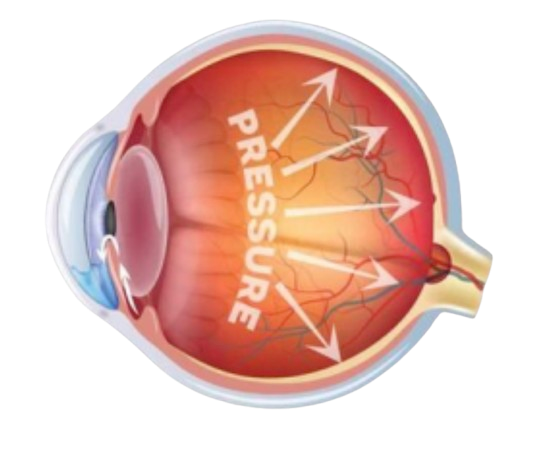
So, what exists?
Prescribed eyedrops
• IOP-reducing eyedrops have been prescribed for decades
• But patients don't know when IOP is spiking and when to apply drops
• Eye pressure can spike at any time without warning
($2500+) Diagnostics
• IOP diagnostics are non-portable, invasive, and expensive
• Physicians only see a snapshot of fluctuations
• Disables necessary frequent monitoring to manage glaucoma
The Result? 1/5 go blind
Who Are We?

JOHN FLANAGAN
Senior R&D Advisor
Ex-Dean of Berkeley's College of Optometry

GEORGE TANAKA
M.D. Strategic Advisor
Glaucoma Specialist & Refractive Cataract Surgeon

DAN KIM
Legal Advisor
Partner Orrick, Herrington & Sutcliffe LLP
Patent-Pending Technology: GlaucoGlasses
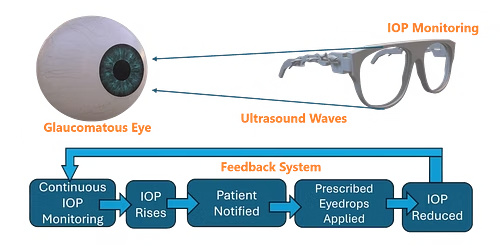
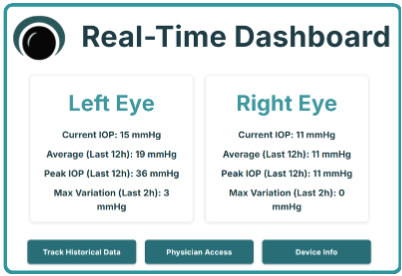
We propose GlaucoGlasses: the world's first ultrasound-integrated smart glasses that continuously monitor eye pressure with 92% accuracy at 95% lower cost than existing devices. With ultrasound transducers and receivers around its frame, Glaucoglasses emits harmless waves to the cornea and analyzes the reflected signal to measure IOP. Mobile notifications prompt patients to apply eye drops when excess eye pressure is sensed to mitigate vision loss. Real-time data is saved to the cloud, enabling remote oversight and treatment personalization from physicians.

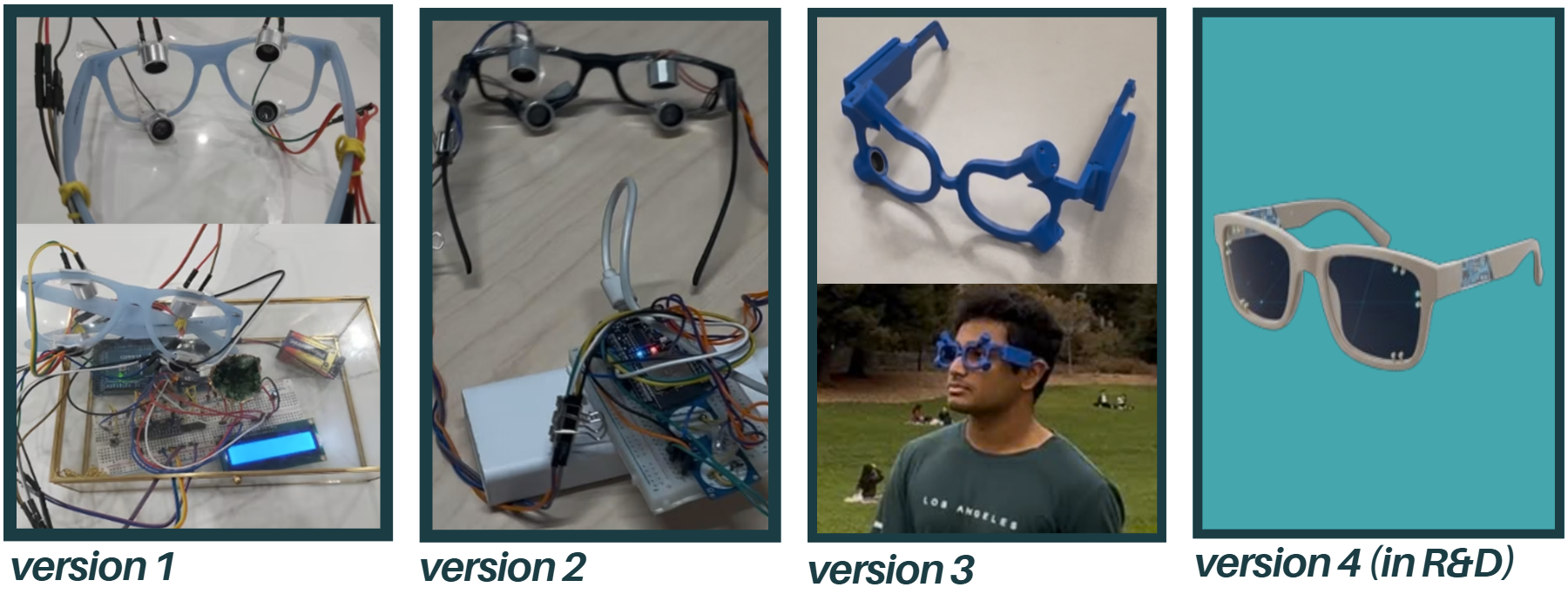
Prototyping Progress: From Bulky Circuits to Sleek PCBs
Our Unique Value
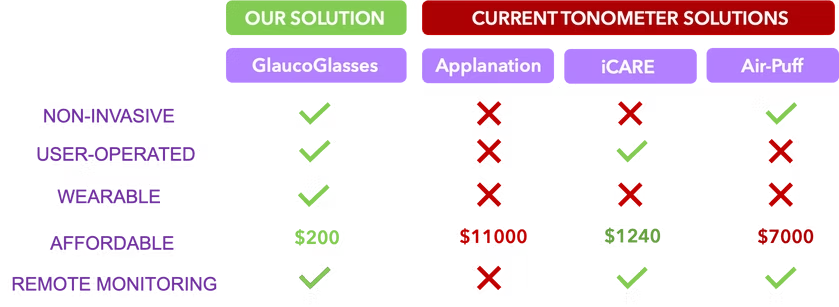
We hold the first wearable and non-contact technology design capable of continuous IOP monitoring, with a 95% cheaper price point and remote physician monitoring feature, outshining each alternative IOP monitor in multiple key factors.
We foresee 3 use cases of GlaucoGlasses: 1) Point-of-Care diagnosis in healthcare settings; 2) Short-term continuous monitoring for baseline IOP level monitoring (for at-risk patients); 3) Long-term continuous monitoring for acute glaucoma and general wellness.
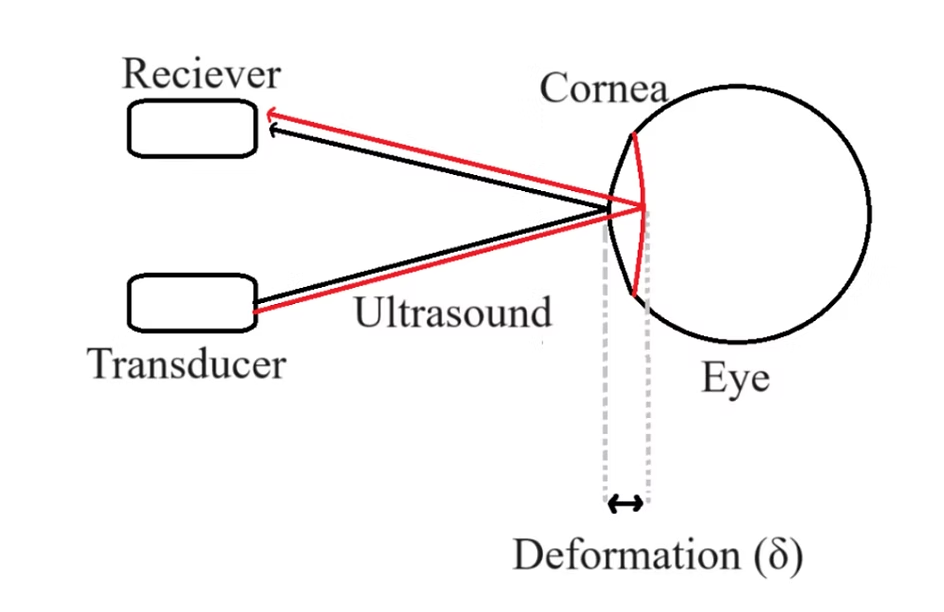
The Science Behind GlaucoGlasses
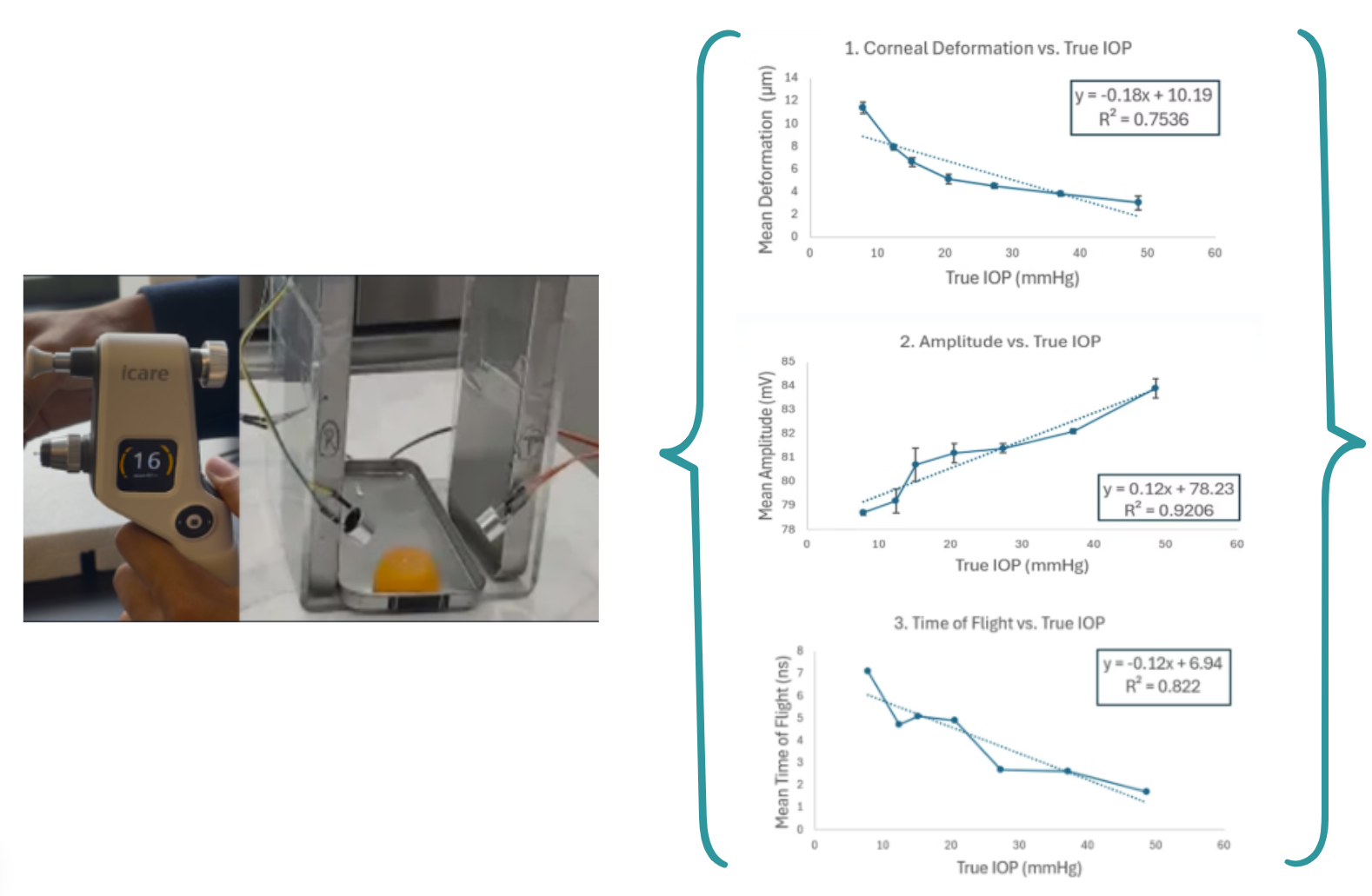
The proof of concept was tested on gelatin phantom eye models under Dr. John Flanagan's purview at Berkeley's College of Optometry. True IOP values were calculated using an iCARE IC100 Tonometer and statistically correlated to the extracted ultrasound signal. Proprietary ML models have been built on this correlation.
Widely used in elasticity imaging, acoustic radiation force from ultrasound waves induces physical deformation in tissues--elucidating data about the elasticity of the tissue. Applying a known magnitude of acoustic force to the cornea of the eye will similarly enable the measurement of corneal deformation, offering insights into elasticity, which is known to be correlated to IOP.
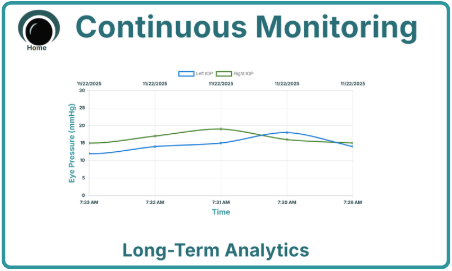
20+ parameters will be experimentally extracted from the reflected ultrasound signal, which will correlate to IOP. The experimental correlation of three of these parameters is shown below.
Contact Us
Ready to revolutionize your eye health? Get in touch with our team to learn more about GlaucoNova Labs and our innovative technology.
Get In Touch
sohamchakraborty03@gmail.com
Phone
+1 (510) 320-6984
Address
123 Innovation Drive
San Francisco, CA 94105

